Cases
Development of CAR-T Drugs
Backgrounds:
Chimeric Antigen Receptor T-Cell Immunotherapy (CAR-T therapy) is an emerging cell-targeted therapy for treating tumors. It involves engineering T cells isolated from a patient's body to express chimeric receptors that target specific antigens. The modified CAR-T cells can recognize and kill tumor cells with the targeted antigen specifically, achieving the goal of treating the tumor. In August 2017, Kymria, a CAR-T therapy developed by Novartis, became the first CAR-T drug approved by the FDA for treating B-cell acute lymphoblastic leukemia (ALL). In June 2021, Axicabtagene Ciloleucel Injection, a CAR-T therapy developed by FOSUN Kite, became the first domestically approved CAR-T drug in China. The emergence of multiple CAR-T drugs on the market indicates that CAR-T therapy development has entered a fast lane, and the relevant industry is entering a rapid expansion period. ABLINK provides customers with high-quality and patent-risk-free CAR-T discovery services using its independent antibody library and CAR-T library.
Procedure:
ABLINK's large-molecule drug library construction and screening services meet the customer's needs for discovering high-affinity antibodies, peptides, and ligands. At the same time, ABLINK has mature T cell display technologies. Different types of antibodies (IgG or nanobody), antibody fragments (VHH, scFv, or Fab), novel antibodies (scIgG or scFab), peptides, and ligand protein mutation libraries are displayed on the surface of T cells through lentiviral infection to form the CAR-T libraries. Then, 3-5 rounds of high-throughput panning are carried out using cell sorting technology to select CAR-T monoclonal antibodies that can activate T cells with the properties of high specificity, high affinity and expression stability. These monoclonal antibodies can be directly used for subsequent animal studies and clinical trials.
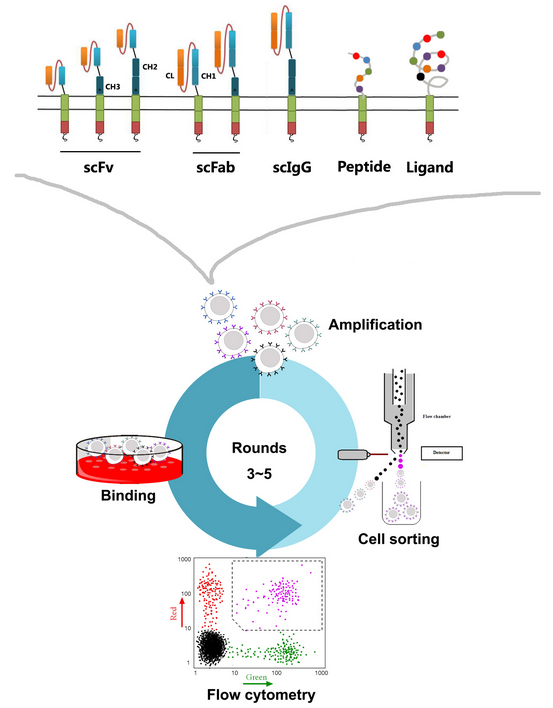
Service Advantages and Features:
1. A complete one-stop CAR-T discovery service, from the construction and screening of antibody libraries to the construction and screening of CAR-T libraries, and ultimately providing multiple pre-clinical candidate molecules. ABLINK also has multiple pre-made CAR-T libraries for customers to choose from.
2. Rich CAR-T development experience. ABLINK's anti-BMCA CAR-T drug has entered Phase I clinical trials.
Cases:
BCMAplus: A New Generation BCMA CAR-T Optimized with Chemokine Receptor
BCMA (B cell maturation antigen) target is a protein expressed mainly in plasma cells and mature B cells. BCMA is a selectively expressed receptor in multiple myeloma (MM) cell lines, but is almost undetectable in healthy human cells, making it an ideal therapeutic target. ABLINK's new generation BCMA CAR-T drug, BCMAplus, has entered Phase I clinical trials at West China Hospital. BCMAplus has enhanced tumor targeting ability (Fig.C, Fig.E), and only uses 1/10 of the conventional CAR-T cell dosage to effectively eliminate tumors (Fig.D).

